FDA approves injectable PrEP medication that’s more effective than Truvada
PrEP injections every two months are more effective at lowering the risk of HIV transmission than daily pills, trials found

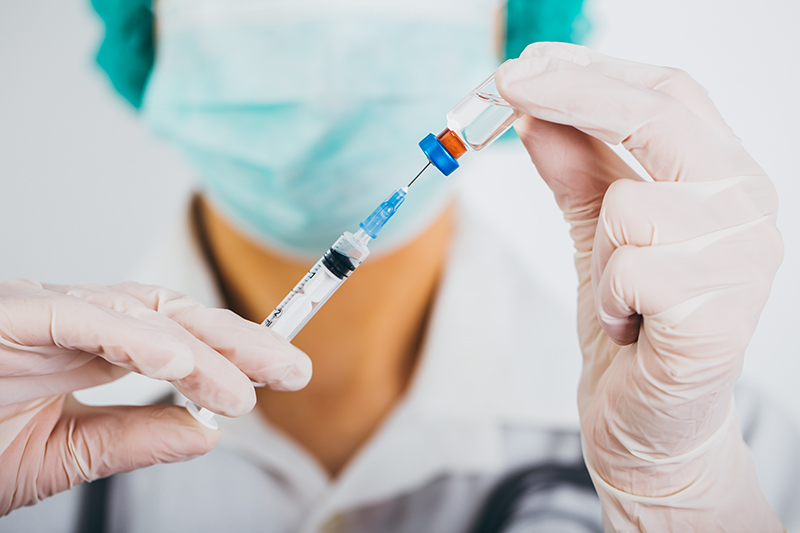
The US Food and Drug Administration has approved a form of PrEP that requires injections every two months, rather than a daily pill.
PrEP, or pre-exposure prophylaxis, uses antiretroviral medications used to treat HIV to combat the virus in HIV-negative individuals.
ViiV Healthcare’s Apretude has been approved for use in at-risk adults and adolescents weight at least 77 pounds, the FDA announced this week. Treatment begins with two shots, one month apart, and then every two months after that.
“Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill,” said Debra Birnkrant, M.D., director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research.
“This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option.”
Prior to Apretude’s approval, PrEP was only available in the form of once-daily pills, sold under the brand names Truvada and Descovy.
While daily PrEP pills are up to 99% effective at preventing transmission of HIV during sexual intercourse, trials found that ViiV’s Apretude injections were even more effective at lowering the risk of transmission.
Related: Health insurance must cover all costs associated with PrEP, Biden administration orders
A trial of the injectable treatment was so successful that it was stopped early, and the group being prescribed Truvada offered Apretude instead.
“People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the US, may want options beyond daily oral pills,” ViiV Healthcare CEO Deborah Waterhouse said in a statement.
“That’s why ViiV Healthcare is proud that Apretude was studied in one of the most diverse and comprehensive HIV prevention trial programs to date, which also included some of the largest numbers of transgender women and Black men who have sex with men ever enrolled in an HIV prevention trial. With Apretude, people can reduce the risk of acquiring HIV with as few as six injections a year.”
Waterhouse added: “Today’s approval is the latest example of ViiV Healthcare’s commitment to developing long-acting medicines that offer consumers a different choice.”
Related: Injectable HIV treatment only requires six doses per year, study finds
The FDA reports that of the 1.2 million people in the U.S. for whom PrEP is recommended, 25% are currently being prescribed it. That compares with 3% in 2015.
The agency noted that daily PrEP “requires high levels of adherence to be effective and certain high-risk individuals and groups, such as young men who have sex with men, are less likely to adhere to daily medication.”
“It is hoped that the availability of a long-acting injectable PrEP option will increase PrEP uptake and adherence in these groups,” the FDA said.
ViiV Healthcare, a specialist HIV company which is part of GlaxoSmithKline, received FDA approval earlier this year for Cabenuva, a monthly injectable antiretroviral regimen to treat HIV.
The monthly injections were touted for their ability to reduce treatment for the virus from a daily pill regimen to just 12 treatment days per year.
However, a subsequent study found that Cabenuva can achieve similar levels of performance when administered every two months.
Related:
HIV advocates praise Biden administration’s “refocused” national HIV/AIDS strategy
Biden recognizes World AIDS Day, highlights impact of HIV on LGBTQ community
PrEP, testing, and treatment as prevention leads to 80% drop in new HIV diagnoses
Read More:
Air Force authorizes use of pronouns, including gender-neutral ones, in official emails
DC gay bars will require proof of coronavirus vaccination for entry
Utah billionaire severs ties with Mormon church, accuses it of “actively” harming LGBTQ rights
Support Metro Weekly’s Journalism
These are challenging times for news organizations. And yet it’s crucial we stay active and provide vital resources and information to both our local readers and the world. So won’t you please take a moment and consider supporting Metro Weekly with a membership? For as little as $5 a month, you can help ensure Metro Weekly magazine and MetroWeekly.com remain free, viable resources as we provide the best, most diverse, culturally-resonant LGBTQ coverage in both the D.C. region and around the world. Memberships come with exclusive perks and discounts, your own personal digital delivery of each week’s magazine (and an archive), access to our Member's Lounge when it launches this fall, and exclusive members-only items like Metro Weekly Membership Mugs and Tote Bags! Check out all our membership levels here and please join us today!































You must be logged in to post a comment.